Description:
Invetion Reference: 11/MED/454
|
There are over 10 million new cases of cancer every year. To fight this global health challenges, new treatment approaches, besides traditional strategies like chemotherapy and radiotherapy, are urged. Gene therapy is one of the promising approaches, but a safe and efficient delivery method to tumor site is necessary for its bench to bed side development. Mesenchymal stem cells (MSCs) are found in many fetal and adult tissues and can generate new cells either continuously during tissue development or remodeling or in response to injury stimuli. Tumor is considered as wounds that never heal, and tumor microenvironments have many similarities with the tissue repair processes that attract specific migration of MSCs (homing). |
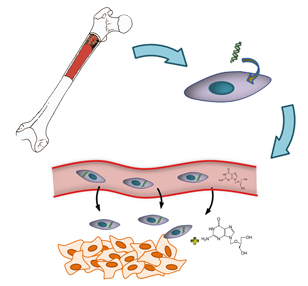 |
Stem/progenitor cells of human origin have been shown to migrate to multiple tumor types, including glioblastoma, melanoma pancreatic and breast carcinoma, regardless the location of the tumors. With its low immunogenicity and homing properties, MSCs represent a good candidate as anti-tumor agent delivery vehicle which also solve inherent gene therapy delivery problems. The potential use of effective and tumor-selective MSCs-based therapies for in vivo delivery of various clinically relevant antitumor agents, including cytokines, interferon, prodrugs, oncolytic virus, or anti-angiogenic agents have been reported. This invention provides a versatile cell-based anti-tumor vector derived from immortalized human mesenchymal stem cell for the delivery of suicide gene, herpes virus thymidine kinase, to tumor site. The well-characterized property, high passaging capacity and sterility scanning in advance make this invention an off-the-shelf product and available for emergency use.
Gene therapy employing MSCs overexpressing herpes simplex virus thymidine kinase against maglinant glioma has been reported. In general, administration of a prodrug, ganciclovir (GCV), to TK-MSCs injected animals could be converted by the thymidine kinase into cytotoxic product and thus exhibit direct and bystander anti-tumor effects. However, the clinical use of genetically modified MSCs is hampered by its low passaging capacity. A higher passaging capacity can be achieved by gene modification with oncogenes like SV40 large T antigen (TAg) of this invention. TAg is a well characterized immortalizing gene which can immortalize cells in the absence of other oncoproteins and induce cell growth, although there is concern about its associated turmorigenicity. Fortunately, this potential risk could be eliminated during the treatment because the SV40-TK-MSCs will be killed by the cytotoxic product as well.
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |
|
|
|
Inventors:
Keywords:
|